Due to the epidemic situation abroad, some certification bodies have stopped issuing certificates, and the price of protective equipment certification services has also increased, rising from several thousand yuan to more than 10,000. Soaring export demand also brings corresponding risks.
At present, many unqualified intermediary agencies use enterprises' ignorance of foreign regulations to mislead enterprises to make wrong choices and issue invalid certifications, which makes enterprises face huge commercial risks in the process of exporting to foreign countries.
Recently, the customs seized exported medical supplies that did not obtain a medical device product registration certificate.
At the same time, export enterprises are reminded to check the authenticity of relevant certifications before customs declaration to avoid losses.
Key points:
It is important to distinguish the authenticity of the certificate!
Below, China Unicom and you will learn how to distinguish the authenticity of the mask certification.

Guangzhou Baiyun Airport Customs, a subsidiary of Guangzhou Customs, seized a batch of 100,000 medical masks declared as "non-medical disposable protective masks", and the manufacturing enterprise did not obtain a registration certificate for medical device products in my country.
1. China
Medical masks belong to the second category of medical devices in China, and are registered and managed by the provincial drug regulatory department. You can check the medical device access number by checking the medical device.
First open the official website
http://www.nmpa.gov.cn/
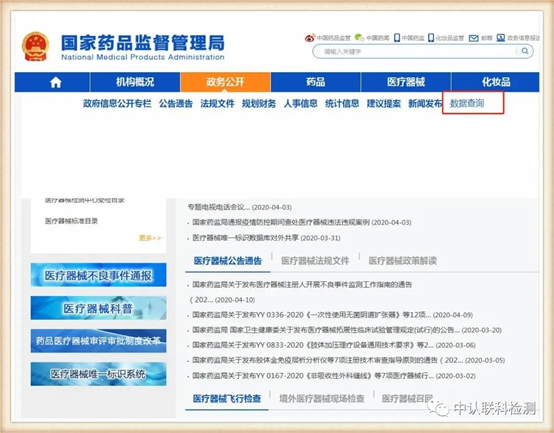
Select the data query under the "Public Affairs" column
http://www.nmpa.gov.cn/
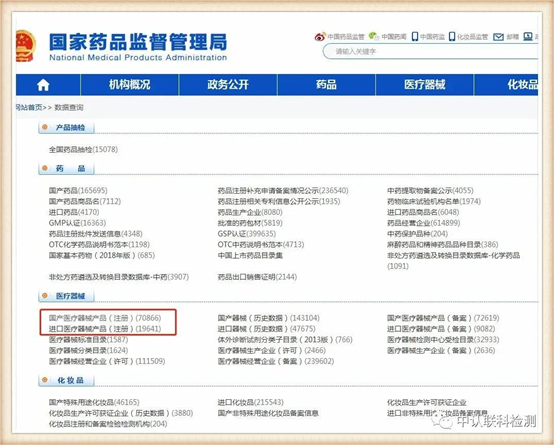
After entering, select as needed in the column of medical devices
http://www.nmpa.gov.cn/
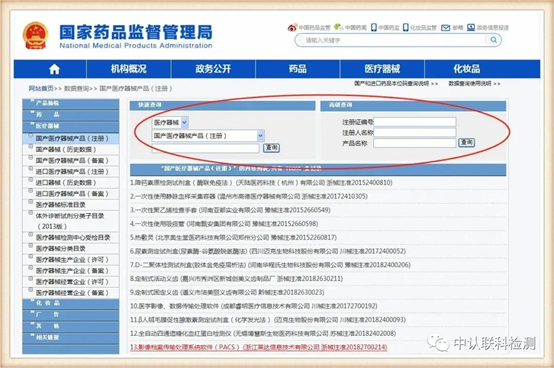
After entering this page, according to the situation marked above, enter the product information indicated on the minimum independent packaging of the product to query.
2. United States
Medical masks are medical devices in the United States and are managed by the Food and Drug Administration (FDA). Recently, the FDA personally dispelled rumors and stated on the official website that it will not issue certifications to any enterprise.
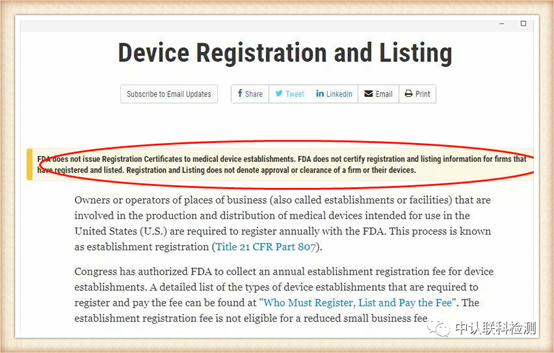
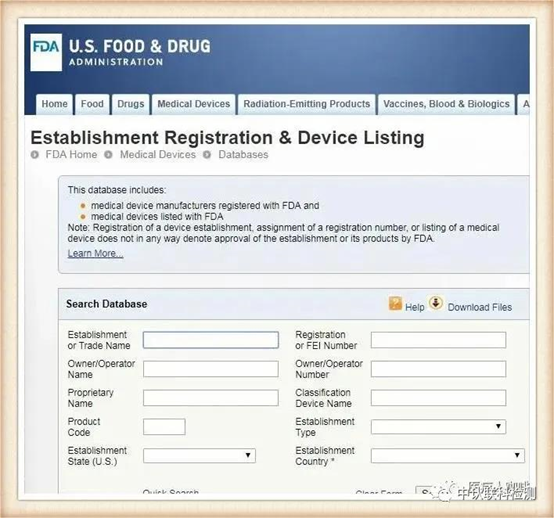
Mask products that have been approved by the US FDA can be checked through the official website for the registration certificate number.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm
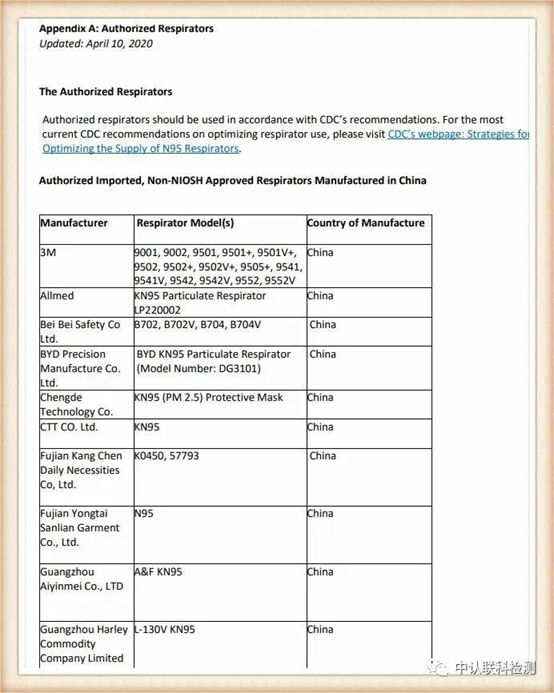
According to the latest FDA policy, it is currently a mask that recognizes Chinese standards when certain conditions are met
The authorized enterprise link is https://www.fda.gov/media/136663/download
3. European Union
The CE certifications on the market are various, almost false. An enterprise applying for certification can ask two questions from the certification authority:
1 Is your company an NB institution? Can the institution number be queried?
2 Can the CE certificate issued be checked on the official website?

(Common CE certification pseudo-certificates, pictures from the network)
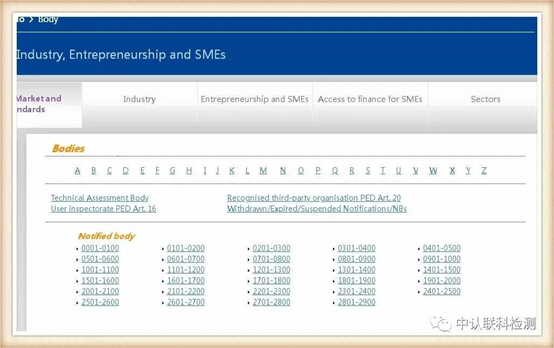
The European Union has announced a series of institutions authorized by the European Union for unified supervision and certification qualifications, which we call NB institutions, and granted each institution a unique four-digit code, that is, the announcement number. The application and issuance of CE certificates are governed by the corresponding regulations And issued by the agency authorized by the announcement number.
The link is as follows
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=notifiedbody.main
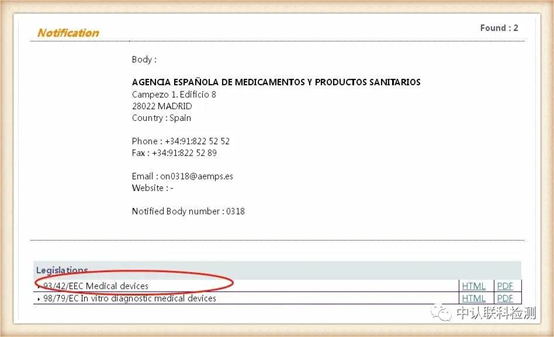
Corresponding to the obtained NB authorization number, click the position of the corresponding code, after entering, you can query the institution's authorized instruction. The certificate issued by the instruction within the scope of authorization is valid. The current EU and mask-related directives are: Medical Device Directive 93/42/EEC (MMD), New Regulations for Medical Devices (EU) 2017/745 (MDR), Personal Protective Equipment (PPE) Regulations (EU) 2016/425.
4. South Korea
Medical surgical masks are class II medical device products in Korea. MFDS (Korean Ministry of Food and Drug Safety) implements pre-market approval for Class II devices. South Korea stipulates that importers of such commodities should ensure that imported products comply with the requirements of the quality management system and obtain permission from MFDS authorized institutions. MFDS only issues certificates to domestic companies. Han Dynasty has a heavier duty, so don’t worry too much about the authenticity of the certificate.
5. Australia
Mask products in Australia need to be registered by the Australian Australian Government Health Products Authority TGA. TGA is the supervision agency for Australian therapeutic products (including drugs, medical devices, genetic technology and blood products), according to Australian Therapeutic Goods (Medical Devices) Regulations 2002, Australia's classification of medical devices is almost the same as the EU classification. If it has obtained a CE certificate issued by the EU Notified Body, it can be recognized by TGA and can be used as important registration information to meet Australian safety regulations. After the TGA is approved, the ATRG registration number will be generated. The query method is as follows.
First, enter the official website http://www.tga.gov.au/
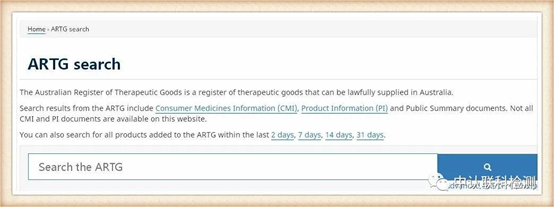
Select "Australian Register of Therapeutic Goods (ARTG)" in the box to start searching
http://www.tga.gov.au/
ZRLK provides customers with personal protective masks, N95 masks and other CE certification, EUA certification, REACH testing, domestic quality inspection reports and other mask export needs documents, professional engineers to answer your questions, if you have any questions or unclear, please contact our company , Enterprises in need can directly contact ZRLK to consult related businesses.